
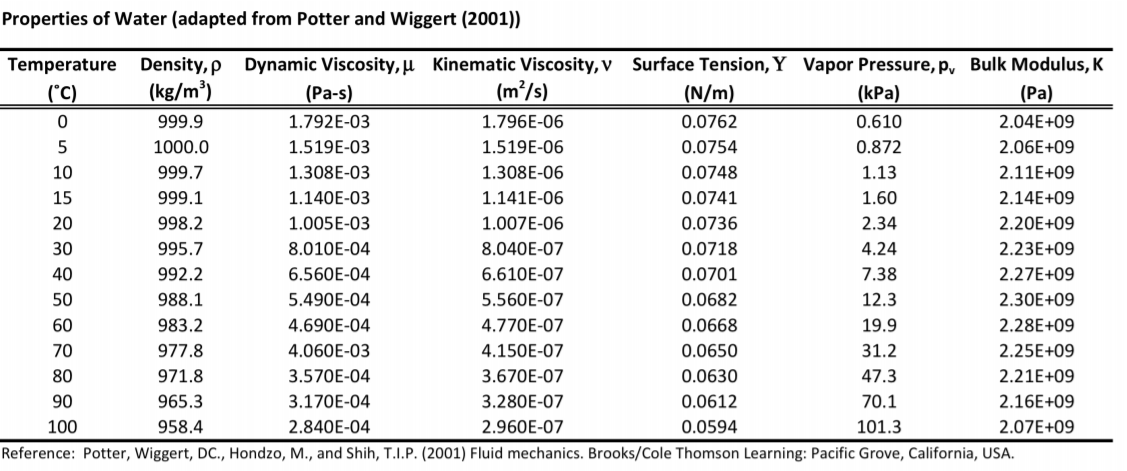
Dolan, Willey, 2010, 3rd ed.).įor different solvents look in the Mixtures of Water and Organic Compounds file in Landolt-Börnstein Database. These data are based on the table presented in Introduction to Modern Liquid Chromatography (L.R. Further, as you can see from the following figures, the viscosity decrease with higher temperature, which forces further research in the field of high temperature liquid chromatography. Generaly, the viscosity decreases with increase in concentration of the organic modifier, acetonitrile or methanol, respectively. Thus, higher flow rates of the mobile phase can be used and lead to the shorter analysis time.įollowing tables and plots show dependence of the viscosity for a acetonitrile-water and a methanol-water mixtures at different composition of the binary solvent and temperature. Clearly, the mobile phase with lower viscosity shows lower instrumental pressure. In liquid chromatography, the viscosity of the mobile phase plays crucial role. The SI unit for the viscosity is Pascal-second (Pa.s).ġ cP = 0.001 Pa.s Viscosity of the mobile phase Viscosity of water taken from Properties of Ordinary Water-Substance. The unit of viscosity is called the poise (P). Viscosity of Aqueous Glycerine Solutions in Centipoises/mPa s. More technically speaking, viscosity is classically defined as the tangential force per unit area necessary to maintain unit relative velocity between two parallel plates in a liquid unit distance apart. For example, water is “thin” with low viscosity, while honey is “thick” having a higher viscosity (see example on right). In case of liquids, the viscosity can be simply expressed as “thickness”. This resistance is called viscosity and can be expressed as a resistance to flow. UPDATED – One of the general property of the liquids is their resistance to change a form. In Table 2, we present most common units for viscosity and the conversion factors between them.Example of liquids with different viscosity Note that 1 cm 2/s is equivalent to 100 cSt. However, due to the viscosity values of most common fluids, square centimeters per second (cm 2/s) is used more often. The SI unit for kinematic viscosity is square meters per second (m 2/s). One stoke is equivalent to one poise divided by the density of the fluid in g/cm 3. Kinematic viscosity is often measured in the CGS unit centistokes (cSt), which is equivalent to 0.01 stokes (St). You guessed it! This one is named after Irish mathematician Sir George Gabriel Stokes (1819-1903) who, among other contributions to fluid mechanics, helped develop the Navier-Stokes equation for the conservation of momentum. Viscosity of Common Fluids Units for Kinematic Viscosity However, since the viscosity of most fluids is below 1 Pa-s (See Table 1), the millipascal-second (mPa-s) is often used instead. 5600 Lower Macungie Road Macungie, PA 18062. The viscosity of water at a temperature of 20 degrees Celsius is approximately 0.01 poise or 103 Pa. Water 70☏ / 21☌: 1: centipoise (cps) Blood or Kerosene: 10: centipoise (cps). You can always check our application library to find examples of different fluids and their viscosity. The SI unit for dynamic viscosity η is the Pascal-second (Pa-s), which corresponds to the force (N) per unit area (m 2) divided by the rate of shear (s -1). In layman’s terms, viscosity defines a fluid’s resistance to flow. It is not a coincidence that the viscosity of distilled water at 20☌ was used to define 1 cP! In order to give you an idea of the viscosity of some conventional fluids we have collected their viscosities in Table 1. This unit is used in honor of French physicist, Jean Léonard Marie Poiseuille (1797-1869), who worked with Gotthilf Hagen on the widely known Hagen-Poiseuille law which applies to laminar flow through pipes.
The most commonly used unit for dynamic viscosity is the CGS unit centipoise (cP), which is equivalent to 0.01 Poise (P). In this page we briefly discuss the most common units for the two main types of viscosity: dynamic and kinematic. To further complicate things, different applications might use different unit systems such as SI, CGS. Sometimes it can be confusing since there are several types of viscosity, each with their own units. It is sometimes referred to as the poiseuille (Pl). Pascal-second (symbol: Pa·s) This is the SI unit of viscosity, equivalent to newton-second per square metre (N·s m2). Temperature, however, has the greatest impact on viscosity. Sugar, for example, makes water more viscous. We get asked about the units of viscosity all the time. Adding molecules can have a significant effect.


 0 kommentar(er)
0 kommentar(er)
